It’s possible that you have an organ you’ve never heard of. One part mythical, one part elusive, and one part mysterious, and no we are not talking about the appendix. As of 2019, your microbiome has been designated your body’s newest organ (1)! What began in 2008 as the National Institutes of Health Common Fund Human Microbiome Project to sequence and understand the human microbiome has launched a new scientific field dedicated to the understanding of and the link between this new organ and other organ-system functions including metabolism, immunity, and brain function (2, 3). So, what is your microbiome? And if this mysterious organ is so important for your general health, how does it impact our running and how does our running impact it?
What is the Microbiome?
Your body is home to trillions of microbes, including bacteria, fungi, protozoa, and viruses–about 5,000 species of them (1)! Altogether these microbes compose your microbiome. In fact, a healthy microbiome will weigh approximately 1 to 3% of your total body mass, and in quantity outnumbers our own cells with slightly more than 1 microbial cell to every 1 human cell in your entire body (6). The main sites of your microbiome include your skin, oral cavity, gut, and vagina. I think it’s easy to think of these microbes, particularly bacteria and viruses, as “bad invaders.” However, these cells live predominantly in harmony with our own cells. We will be focusing on the gut microbiome as it’s the most abundant and diverse of the microbiome spaces, and it’s also where the scientific literature is focused.
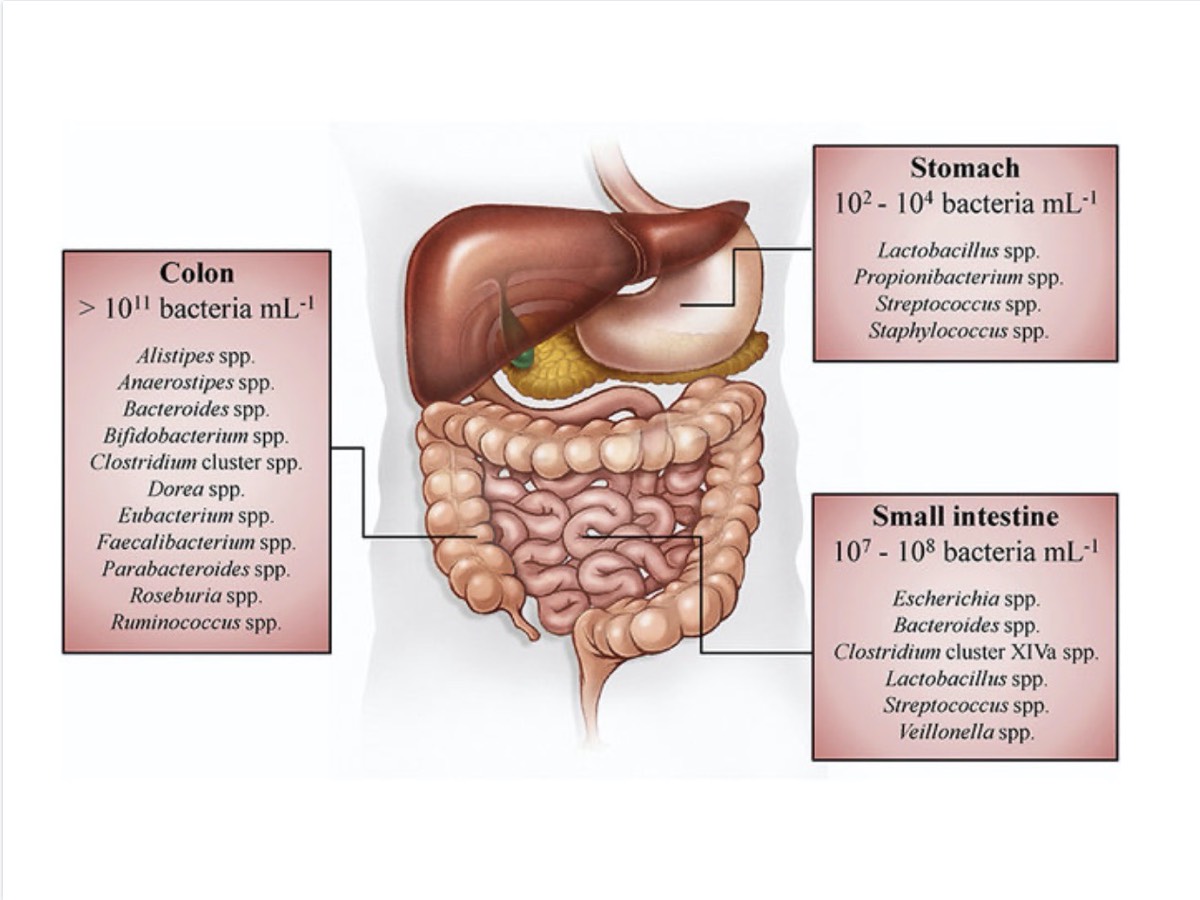
Your small intestine, colon, and stomach are home to thousands of different bacteria species. Your colon is home to greater than 100 billion microorganisms per milliliter of stool, your stomach is home to 100 to 1,000 microorganisms per milliliter of stool, and your small intestine is home to 10 million to 100 million microorganisms per milliliter of stool! Image: Rivière, A., Selak, M., Lantin, D., Leroy, F., & Vuyst, L. D. (2016). Bifidobacteria and Butyrate-Producing Colon Bacteria: Importance and Strategies for Their Stimulation in the Human Gut. Frontiers in Microbiology, 7. doi: 10.3389/fmicb.2016.00979 (5)
These important microorganisms that live in your gastrointestinal (GI) tract play important roles in metabolism, immune function, and even cognitive wellbeing. They do this in many ways including nutrient uptake, the synthesis of vitamins (K and B), harvesting energy from ingested food, and helping to regulate a healthy immune response (7, 4). Another important role of the microbiome is helping with the synthesis of neurotransmitters, specifically serotonin, dopamine, norepinephrine, and gamma-Aminobutyric (GABA). Neurotransmitters are the chemical messengers responsible for regulating many bodily functions like your mood (serotonin and dopamine), increases in heart rate and blood pressure and other stress responses (norepinephrine), and decreasing nervous-system activity (GABA). This role of the microbiome is so important that it is sometimes referred to as the “second brain.” Whereas having an altered gut microbiome in composition or function has been linked to metabolic disorders (obesity and type 1 diabetes), brain and nervous-system-related dysfunctions (depression, anxiety, Alzheimer’s disease, and multiple sclerosis), and GI conditions (Crohn’s and inflammatory bowel disease) (1, 7).
That might sound daunting, but it’s important to know that there is no one perfect microbiome that is the predictor of optimal health. Instead, your gut ecosystem is incredibly individual and highly dynamic. From the time you are born until you are about three years of age, you acquire the core microbial residents of your microbiome which become the community of cells that are unique to you–just like a set of fingerprints (4). From that point forward, you continue to acquire and make shifts in your microbial community but it remains relatively stable. In a study looking at adults in the U.S. over a five-year period, they found that the core approximately 60% of their individual microbiome composition remained stable (8). The other 40% subtly and regularly changes due to multiple intrinsic and extrinsic factors. These factors include age, diet, genetics, environmental exposures (toxins but also exposure to animals, soil, and more), physical exercise, stress, antibiotic use, and even the delivery method in which you entered this world (vaginal birth versus cesarean section).
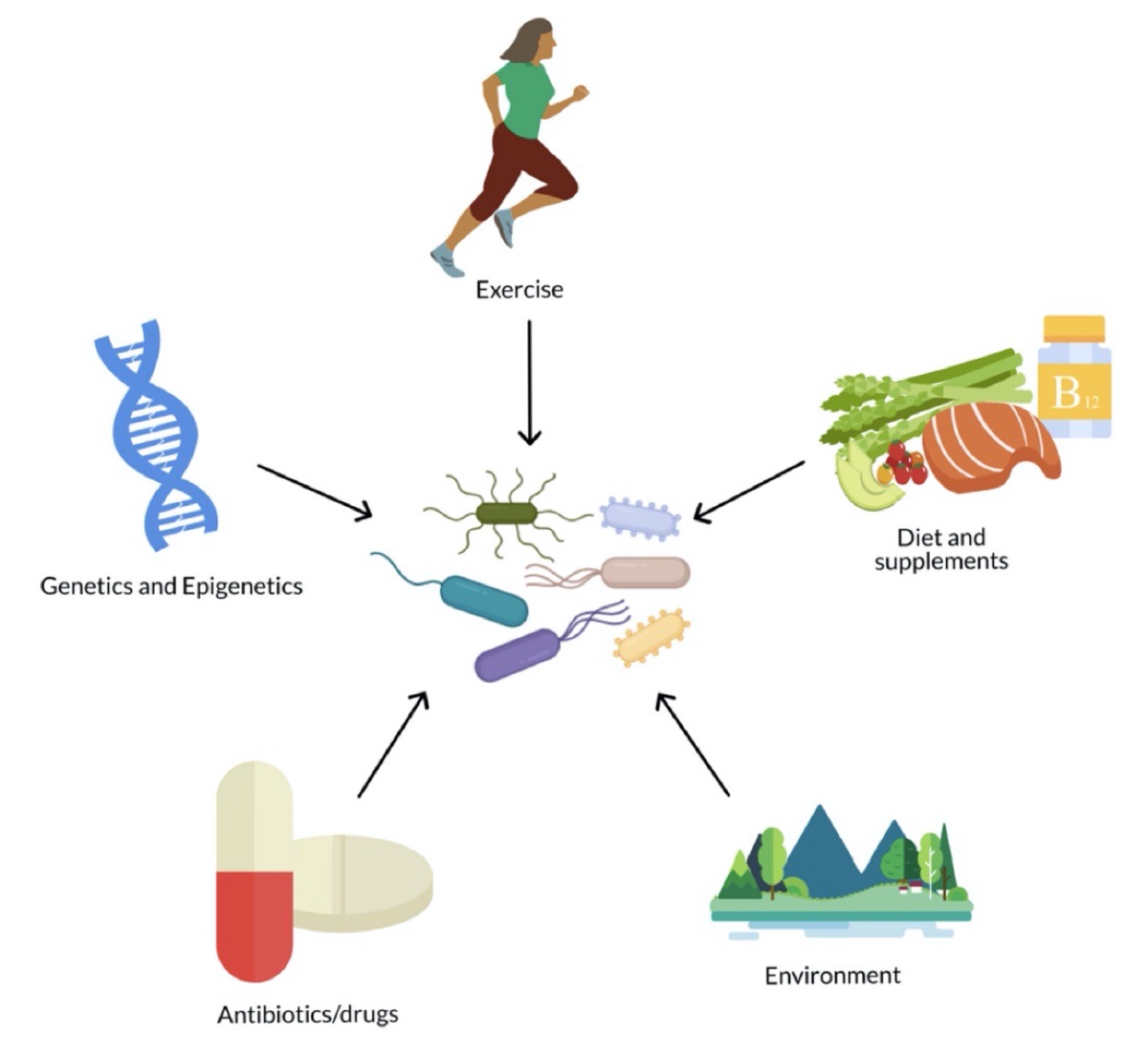
The gut microbiome is influenced by many extrinsic and intrinsic factors. Image: Hughes, R. L. (2020). A Review of the Role of the Gut Microbiome in Personalized Sports Nutrition. Frontiers in Nutrition, 6. Doi: 10.3389/fnut.2019.00191 (3)
What Happens in the Microbiome When We Run?
Exercise is considered one of the main factors for influencing your gut microbiota composition, and generally it’s considered to have the positive effects of increasing both microbiota biodiversity and functionality (10). That’s a good start since high biodiversity is correlated with a healthier microbiome. The functionality piece is interesting. We won’t dive into naming all the types of bacteria that inhabit our community of gut bacteria, but studies in professional and amateur cyclists have shown that the more highly trained endurance athletes had a greater abundance of two species of bacteria associated with greater carbohydrate and amino-acid metabolism and the production of short-chain fatty acids (butyrate, acetate, and propionate) that can be utilized for fuel as well as moderating your inflammatory response (11).
Now, not all exercise is good for the microbiome. As we know all too well, exercise–particularly long and or hard exercise intensities–is a huge physical stressor. As we’ve discussed in our article on GI distress, exercise can induce splanchnic hypoperfusion, or blood flow moving away from the tissues of our digestive tract for use elsewhere in the muscles being heavily used. This change in blood flow induces ischemic (lack of oxygen) events to these important gut tissues which can cause GI distress (abdominal pain, nausea, and diarrhea) and tissue damage including inflammation and GI bleeds (10). This can cause increased permeability of your intestines which puts you at risk for allowing bacteria and potentially toxic byproducts to enter your bloodstream and into systemic circulation (10).
This might not seem like a big deal. I mean, after all wasn’t the bacteria already inside your body? It turns out that, in physiology terms, your GI tract (from your mouth to your anus) is technically “outside.” This means it can interact with other systems (selectively allow nutrients, water, and substrates to be absorbed), but it also protects you from pathogens by remaining an impermeable to semipermeable pipeline. That’s good news, but these moments of increased permeability are great for opportunistic pathogens and terrible for your more dominant bacterial species who you benefit most from that die off and are lost in the process.
How Do We Protect Our Guts?
Enter probiotics and prebiotics. Probiotics are food sources or supplements that contain live microorganisms/bacteria that we ingest to try to colonize or restore the gut flora. Probiotics examples include fermented foods such as yogurt, sauerkraut, and tempeh. Prebiotics are compounds in food that help to induce growth or activity of the bacteria in our microbiome, and examples include fiber-rich food such as fruits, vegetables, and whole grains.
What can these probiotics and prebiotics do to protect our gut besides helping us create and maintain a healthy and diverse microbiome? When we exercise, two things happen to our gut, it gets jostled a lot resulting in inflammation and there is a general decrease in blood flow (splanchnic hypoperfusion) and therefore a decrease in oxygen delivered to the tissue. When this happens, a protein is released called zonulin. This protein is really important because it regulates intestinal permeability by controlling what are known as tight junctions (14). Tight junctions literally hold the adjacent cells that make up the walls of your intestinal tract together. The more zonulin is present, the more permeable your intestinal walls are. When it comes to our intestines, we like them semipermeable, but when there is a lot of zonulin present, this goes too far and makes you susceptible to opportunistic pathogens and GI distress.
However, there have been some studies on the utilization of probiotics and exhaustive exercise and they are showing promising results. When runners ingested probiotics for only 14 days, they had dramatic decreases in fecal zonulin suggesting they were limiting intestinal permeability and thus attenuating possible gut damage (1). However, the results have been mixed at lower intensities–a potentially important note for ultrarunners–and often utilize rodent test subjects, so more research on timing and at varying intensities in human subjects still needs to be done (10).
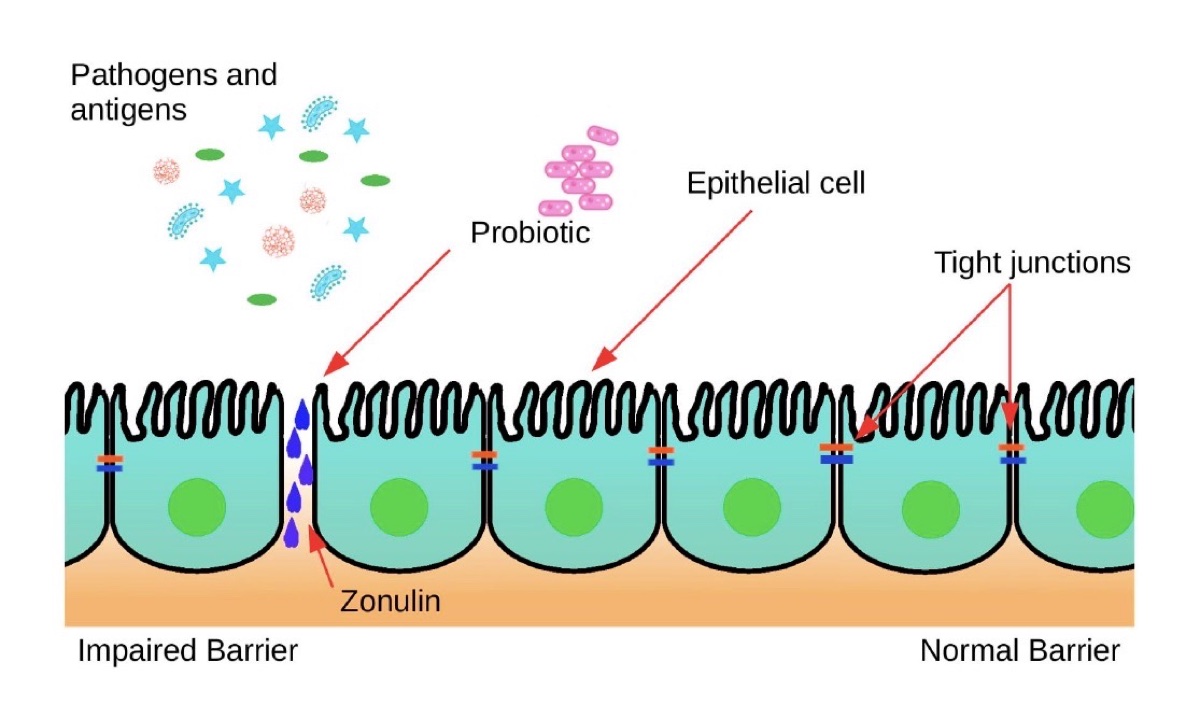
During intense exercise, the protein zonulin is produced at larger rates. Zonulin acts on the GI tract lining’s tight junctions, which hold adjacent cells together, causing “leaks” between them. Probiotics can decrease the amount of zonulin produced, thereby limiting the risk of an impaired intestinal lining. Image: Wosinska, L., Cotter, P. D., O’Sullivan, O., & Guinane, C. (2019). The Potential Impact of Probiotics on the Gut Microbiome of Athletes. Nutrients, 11(10), 2270. doi: 10.3390/nu11102270 (1)
Can My Microbiome Help Me Run Better?
One of the most recent splashes in the athletic world when it comes to the microbiome relates to a three-part study that started at the 2015 Boston Marathon (12). In the study, they analyzed runners’ microbiomes by DNA sequencing fecal samples 1 week before and after the marathon. They found that the abundance of the bacteria species Veillonella increased dramatically in the post-marathon samples, while it was virtually nonexistent in samples from sedentary volunteers. Veillonella is a super-interesting species of bacteria that utilizes lactate or lactic acid as its only fuel source. It does this by metabolizing lactate (that moves from the blood into the intestines) anaerobically (without oxygen) to produce the short-chain fatty acid propionate to be further used by our body as fuel. It’s basically energy recycling! It’s important to note here that our levels of blood lactate are not always high. During aerobic exercise, we produce very little. But an exhaustive or high-intensity effort would produce this favorable environment for Veillonella. We essentially create a positive-feedback loop: producing lactate makes a great environment for Veillonella which in return creates something that benefits us.
This study was then repeated by the same research team with ultrarunners and Olympic-level rowers before and after an exercise bout and found similar results, an abundance of Veillonella in the post-exercise measures.
Finally, and most famously, in the third portion of this study a sample of the bacteria from one of the initial marathoners was used to inoculate mice. The mice had a 13% improvement in time to exhaustion in running tests–presumably on the world’s tiniest treadmill–and a decrease in markers of inflammation. All this because the mice were able to more efficiently convert lactate to short-chain fatty acids.
So when are we getting a pill of Veillonella? Companies are actively working on producing such things, which does not come as a surprise with the market for probiotics expected to exceed $67 billion by 2024 (1). That being said, this was a proof-of-concept experiment in mice and still needs to be researched more thoroughly before we start chasing after elite bacterial samples. However, this study moved the needle from correlation to causation. Not only does exercise (combined with years of high-nutrient intake) positively impact your microbiome, but this diverse and therefore metabolically favorable gut microbiota might return the favor in improving athletic performance.

Magda Boulet is the kraut queen! Magda loves fermented food, including making large quantities and varieties of kraut such as sauerkraut and kimchi. Image courtesy of Magda Boulet.
Food for Thought: The Major Microbiome Takeaways
Here are the three major microbiome takeaways for runners:
Long-Term Dietary Practices Matter
Overwhelmingly what seems most important to endurance athletes is a diet rich in fiber, plant protein, carbohydrates, and of adequate calories. This brought about the most diverse gut microbiota (4). Studies on diets differentiating the type and amount of substrate ingestion (animal versus plant proteins, low carbohydrate versus carbohydrate rich, high protein versus high carbohydrate, and more) showed that extreme dietary changes lasting up to three weeks did not impact the composition of the gut microbiota (4). However, specific long-term dietary practices of longer than three weeks do illicit the growth of specific bacteria species which have positive effects.
Probiotics Can Be Helpful
One of the benefits of probiotics seems to be the protection of your intestines, specifically the maintenance of gut permeability. The mucosal layer of your intestines is important for moderating the absorption of nutrients and water while preventing harmful substances from entering your bloodstream. Long and/or high-intensity exercise and its resultant alterations in blood flow (going to your skin and muscles instead) change the state of the tight junctions and increase the gut permeability. This leads to increases in GI distress (both duration and severity) during and after your runs. Probiotics act protectively, allowing the proteins responsible for those tight junctions to maintain their integrity while you run and race. Probiotics have also been shown to benefit the health of the microbiome residents, decreasing the severity and duration of upper respiratory tract infections by modulating the immune system and inhibiting the pathogen colonization (1).
Antibiotics Are Not Risk Free
To be clear, this isn’t an opposition to antibiotics in whole and it is important to work with your health-care provider to make sure you are prescribed the correct medication. However, there is risk associated with taking antibiotics, so it’s important to take them only when it’s medically necessary. Studies have shown that it takes at least 6 weeks for your microbiome to resemble its original state after a course of antibiotics (1). This is because antibiotics do not only act on the bacteria associated with your infection, but they also affect your resident bacteria as well. A study in 2009 followed several patients post-treatment with the antibiotic ciprofloxacin and they found that the course of antibiotics affected the abundance of about one third of the bacterial species (9). In general, they saw a decrease in richness, diversity, and distribution between the species of bacteria that lasted more than 6 months for certain species, and varied amongst the patients.
Call for Comments
Do you make a conscious effort to care for your gut microbiome?
References
- Wosinska, L., Cotter, P. D., O’Sullivan, O., & Guinane, C. (2019). The Potential Impact of Probiotics on the Gut Microbiome of Athletes. Nutrients, 11(10), 2270. doi: 10.3390/nu11102270
- About the Human Microbiome. (n.d.). Retrieved from https://www.hmpdacc.org/hmp/overview/
- Hughes, R. L. (2020). A Review of the Role of the Gut Microbiome in Personalized Sports Nutrition. Frontiers in Nutrition, 6. doi: 10.3389/fnut.2019.00191
- Mohr, A. E., Jager, R., Carpenter, K. C., Kerksick, C. M., Purpura, M., Townsend, J. R., … Antonio, J. (2020). The athletic gut microbiota. Journal of International Society of Sports Nutrition, 17(24). doi: https://doi.org/10.1186/s12970-020-00353-w
- Rivière, A., Selak, M., Lantin, D., Leroy, F., & Vuyst, L. D. (2016). Bifidobacteria and Butyrate-Producing Colon Bacteria: Importance and Strategies for Their Stimulation in the Human Gut. Frontiers in Microbiology, 7. doi: 10.3389/fmicb.2016.00979
- Sender, R., Fuchs, S., & Milo, R. (2016). Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLOS Biology, 14(8). doi: 10.1371/journal.pbio.1002533
- Strandwitz, P. (2018). Neurotransmitter modulation by the gut microbiota. Brain Research, 1693, 128–133. doi: 10.1016/j.brainres.2018.03.015
- Faith JJ, Guruge JL, Charbonneau M, Subramanian S, Seedorf H, Goodman AL, et al. The long-term stability of the human gut microbiota. Science. 2013;341(6141):1237439 https://doi.org/10.1126/science.1237439.
- Relman DA and Dethlefsen L. Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation. PNAS. 2010;108(1). 4554-4561. doi: 10.1073/pnas.1000087107
- Ticinesi, A., Laurentani, F., Tana, C., Nouvenne, A., Ridolo, E., & Meschi, T. Exercise and immune system as modulators of intestinal microbiome: implications for the gut-muscle axis hypothesis. EIR, 2019; 25, 84-95. Microbiome, muscle and immune interconnections.
- Petersen LM, Bautista EJ, Nguyen H, Hanson BM, Chen L, Lek SH, Sodergren E, Weinstock GM. Community characteristicsof the gut microbiomes of competitive cyclists. Microbiome 5: 98, 2017.
- Scheiman J, Luber JM, Chavkin TA, MacDonald T, Tung A, Pham LD, et al. Meta-omics analysis of elite athletes identifies a performance-enhancing microbe that functions via lactate metabolism. Nat Med. 2019;25(7):1104–9
- Karhu, E., Forsgård, R. A., Alanko, L., Alfthan, H., Pussinen, P., Hämäläinen, E., & Korpela, R. (2017). Exercise and gastrointestinal symptoms: running-induced changes in intestinal permeability and markers of gastrointestinal function in asymptomatic and symptomatic runners.European Journal of Applied Physiology, 117(12), 2519–2526. doi: 10.1007/s00421-017-3739-1

